Modules
The modular programming paradigm with which kemXpro was built makes it scalable, horizontally and vertically, to easily adapt to your actual current and future needs, making concrete cost savings and a distinct operational advantage possible.
You can easily decide to extend it progressively by integrating new modules, new legal entities, new users and new languages.
The extreme flexibility of the technology adopted also allows the integration of custom extensions "tailor-made" for your company and business model.
We'll take care of the rest!
Explore the endless potential of the modules that the kemXpro environment can integrate.
Customers Management
Management of active and potential customers that integrates useful overview functions related to projects, supplies, samples, quality control, technical and regulatory documents, ISO procedures.
Interoperable module that can manage continuous and discontinuous data flows to and from external applications (ERP, CRM, MRP, etc.).
Suppliers Management
Management of active and potential suppliers that integrates useful overview functions related to projects, supplies, samples, quality controls, technical and regulatory documents, ISO procedures.
Interoperable module that can manage continuous and discontinuous data flows to and from external applications (ERP, CRM, MRP, etc.).
Human Resources Management
The kemXpro Human Resources module provides integrated management of human capital both inside and outside the organisation.
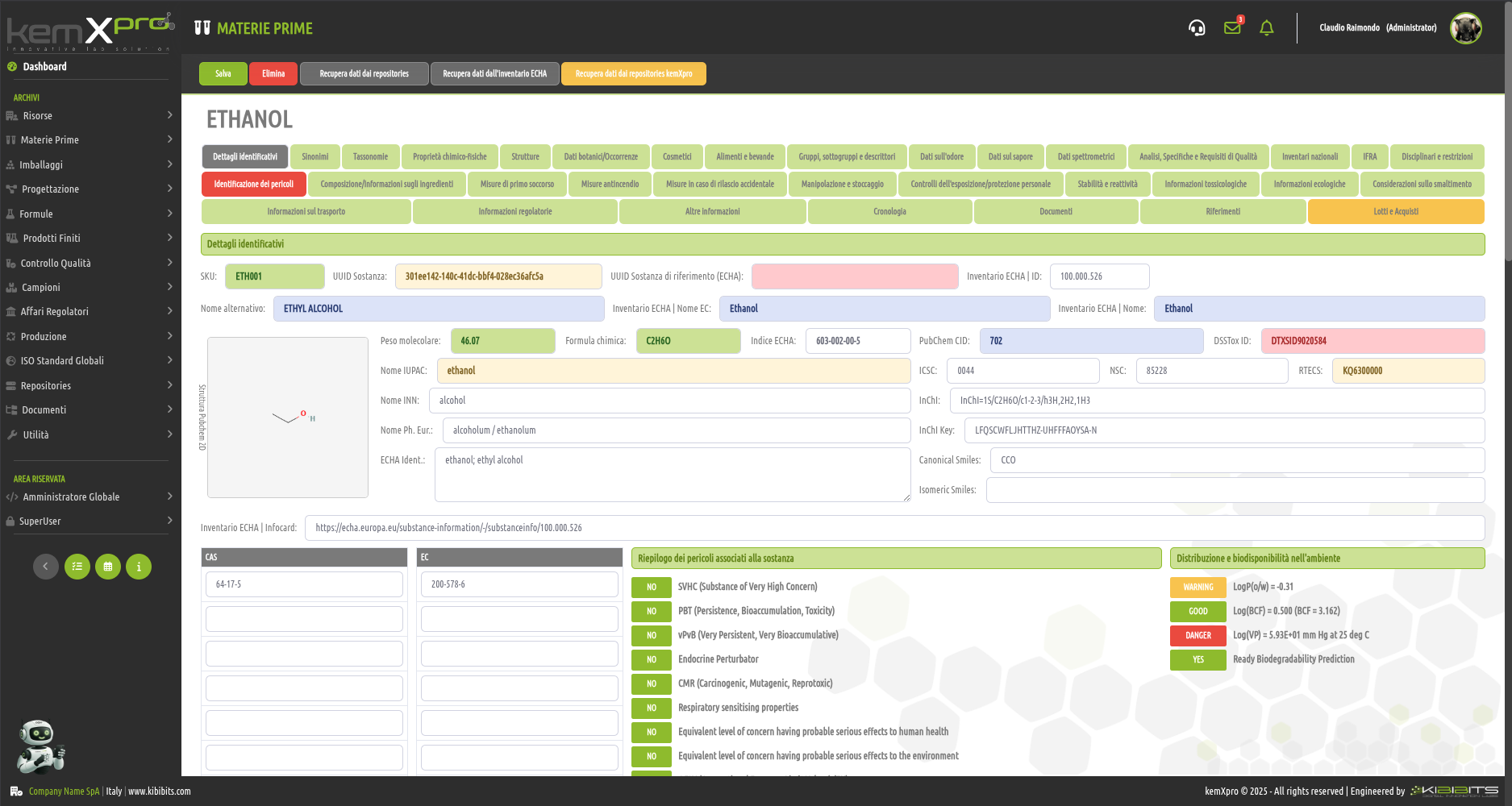
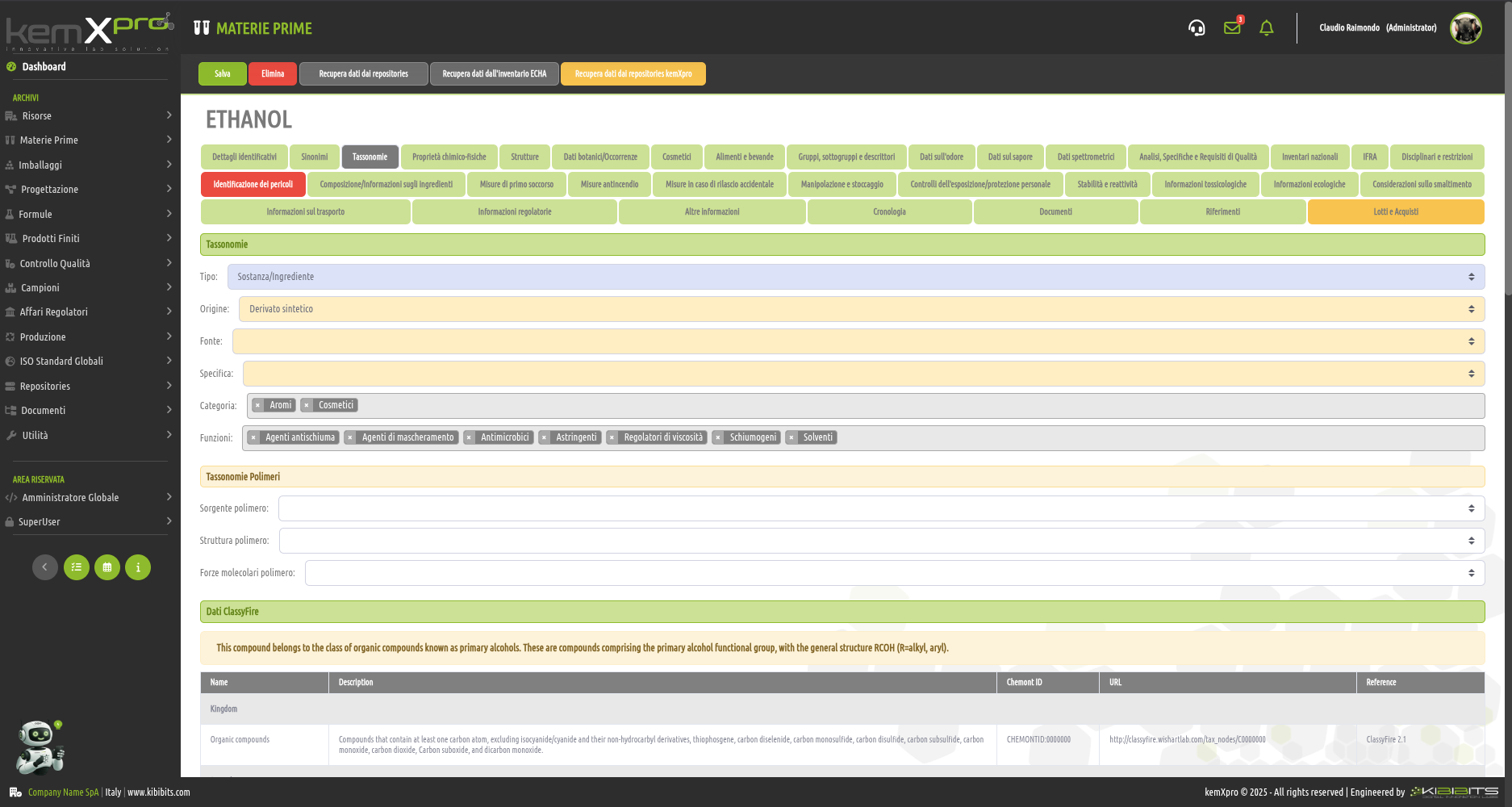
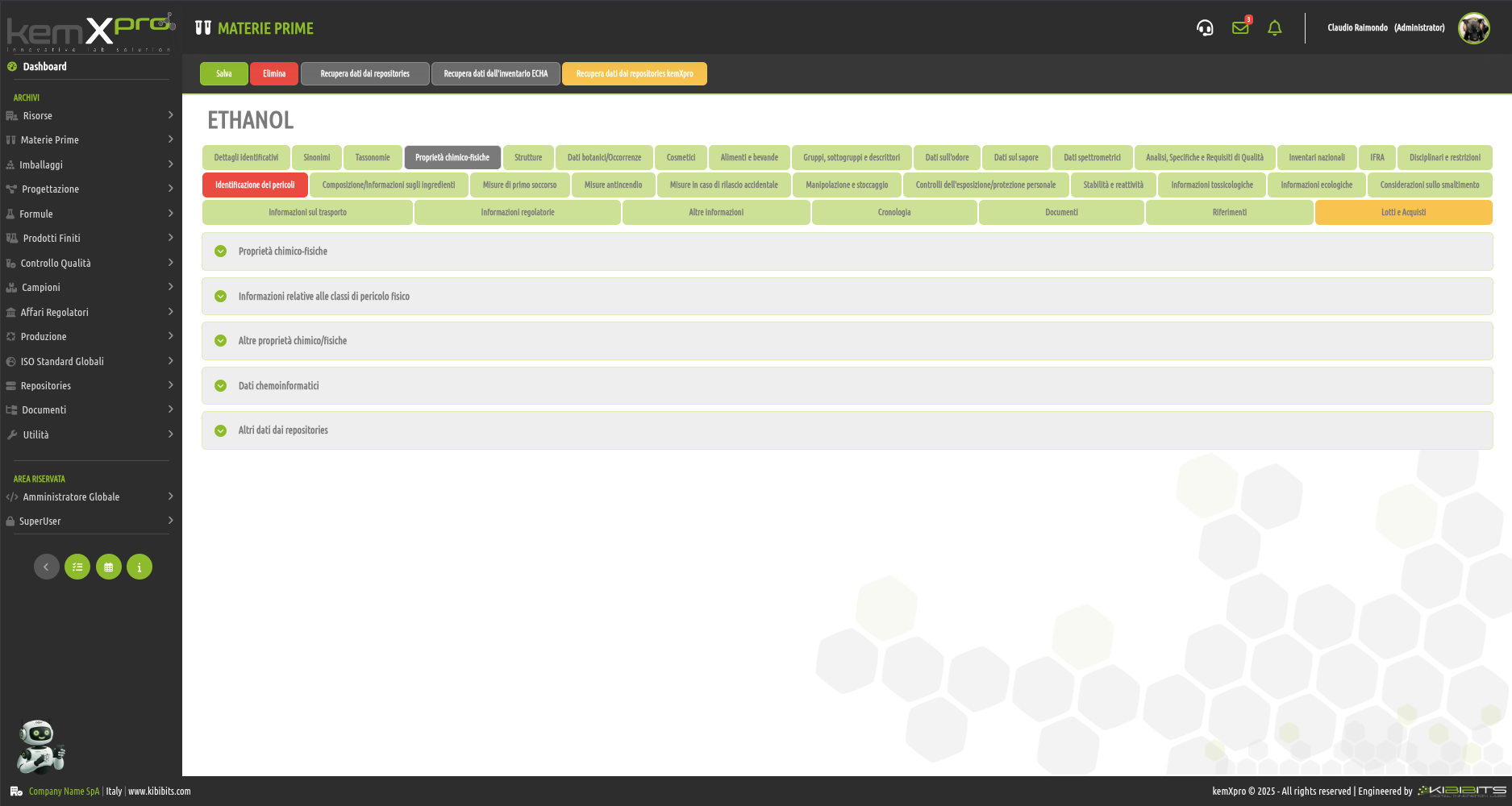
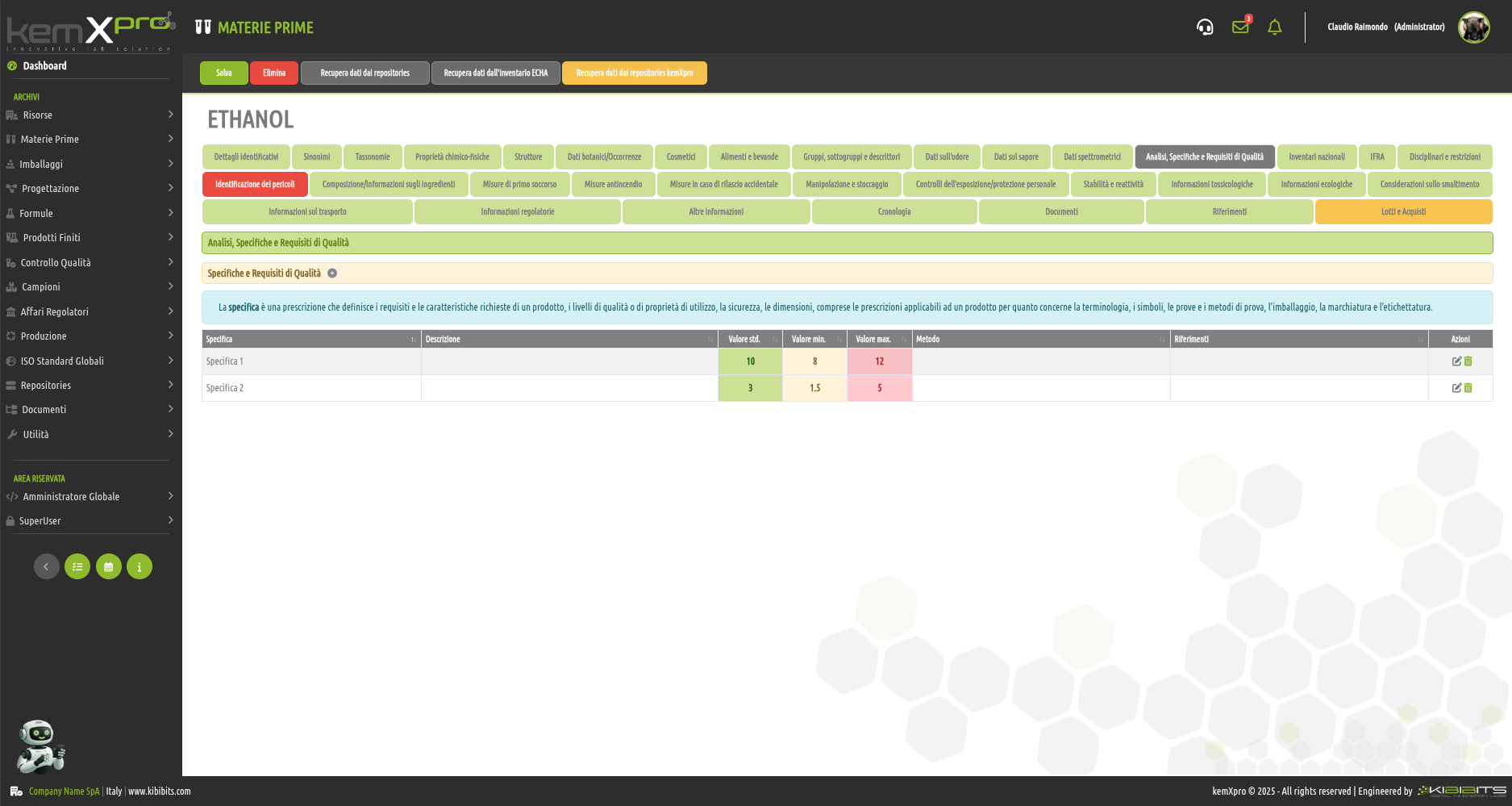
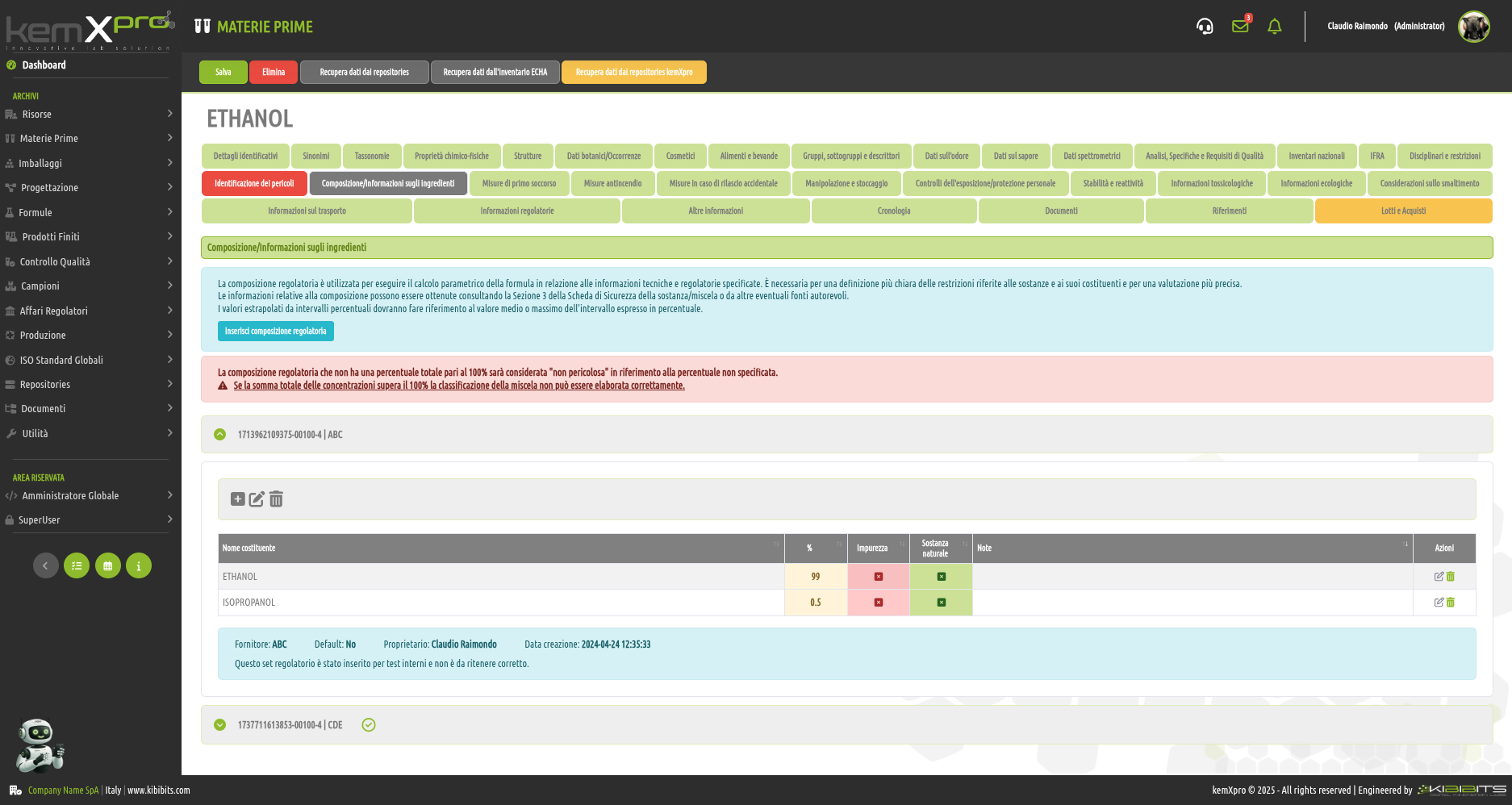
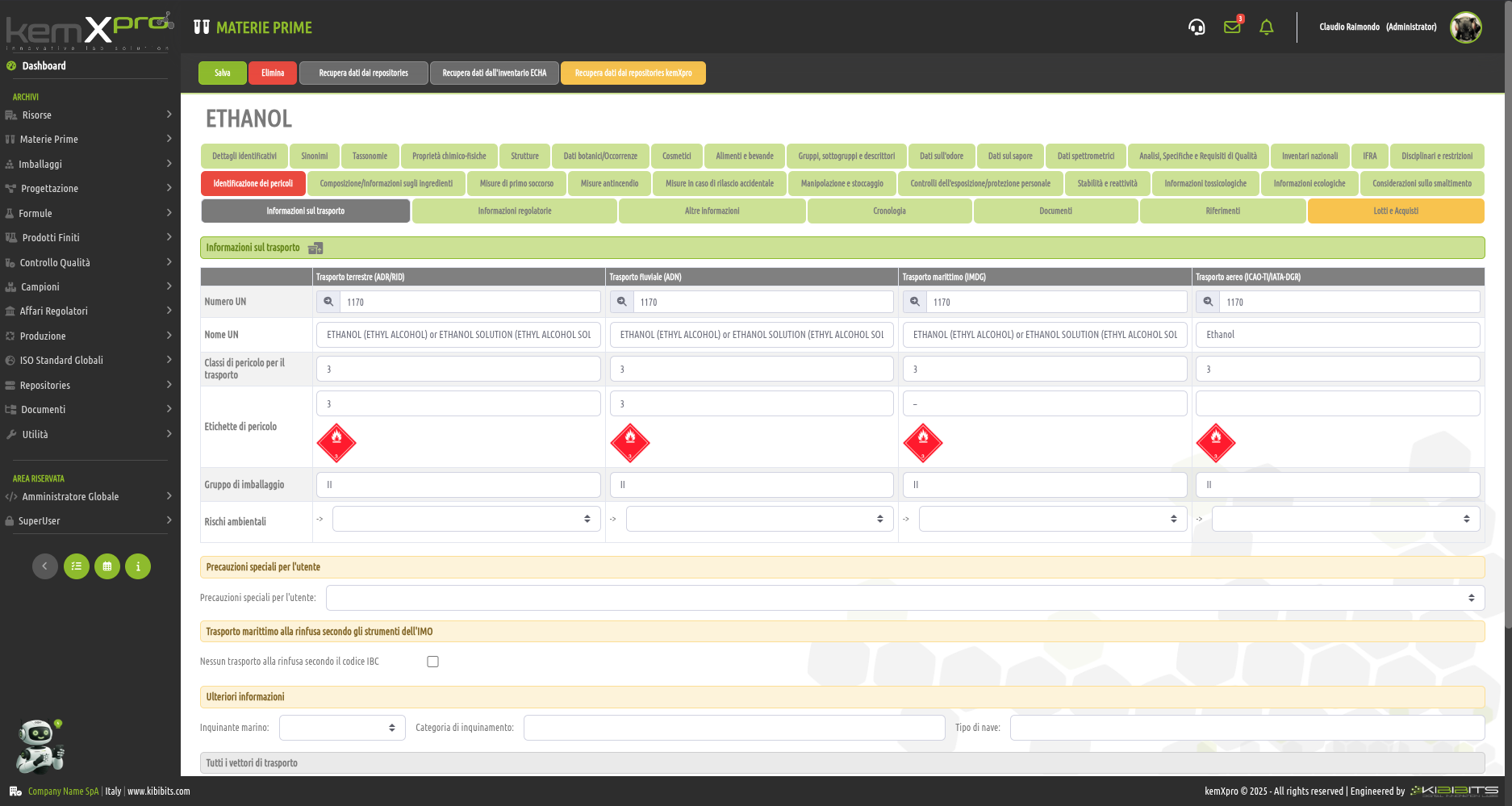
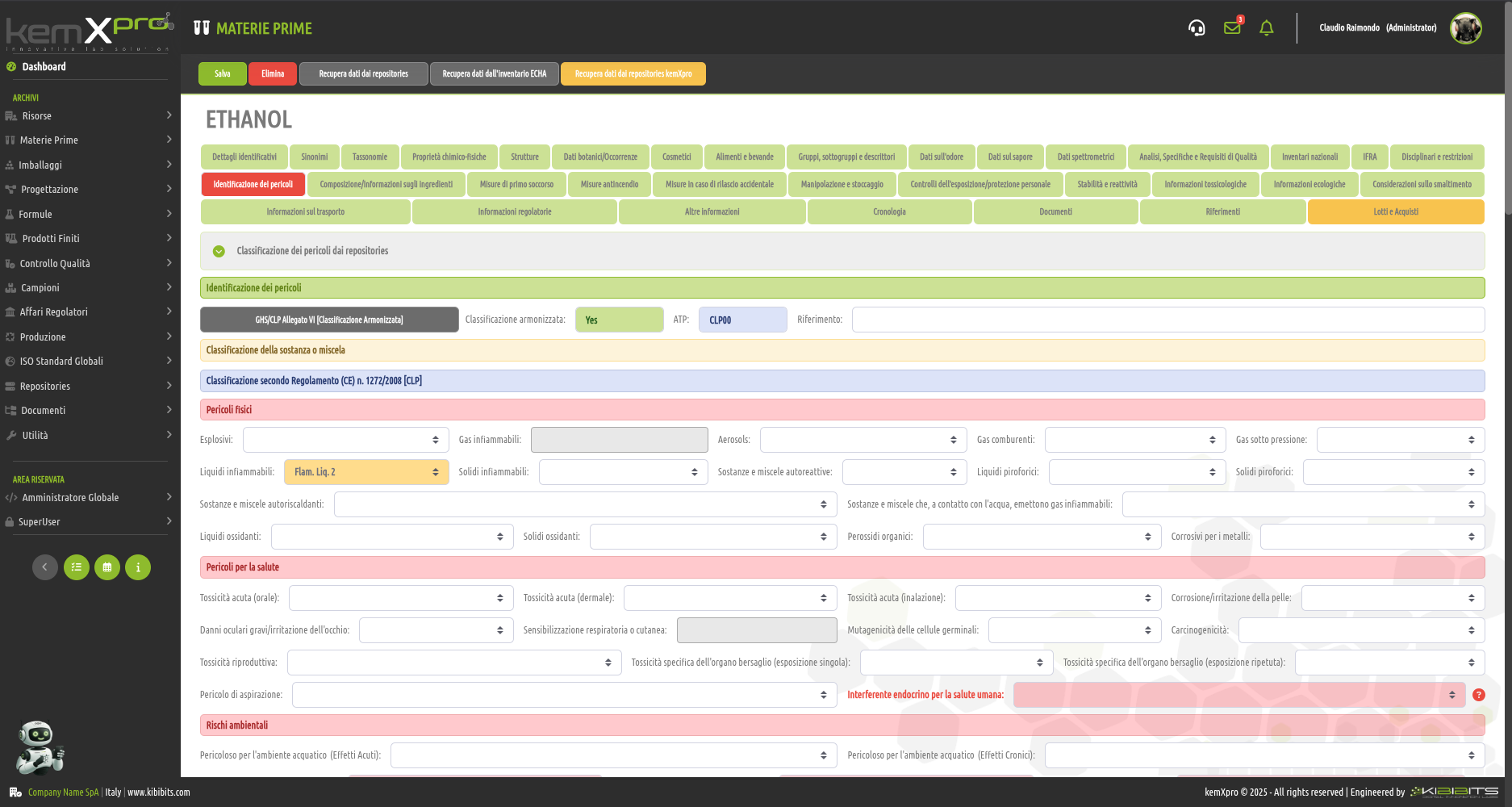
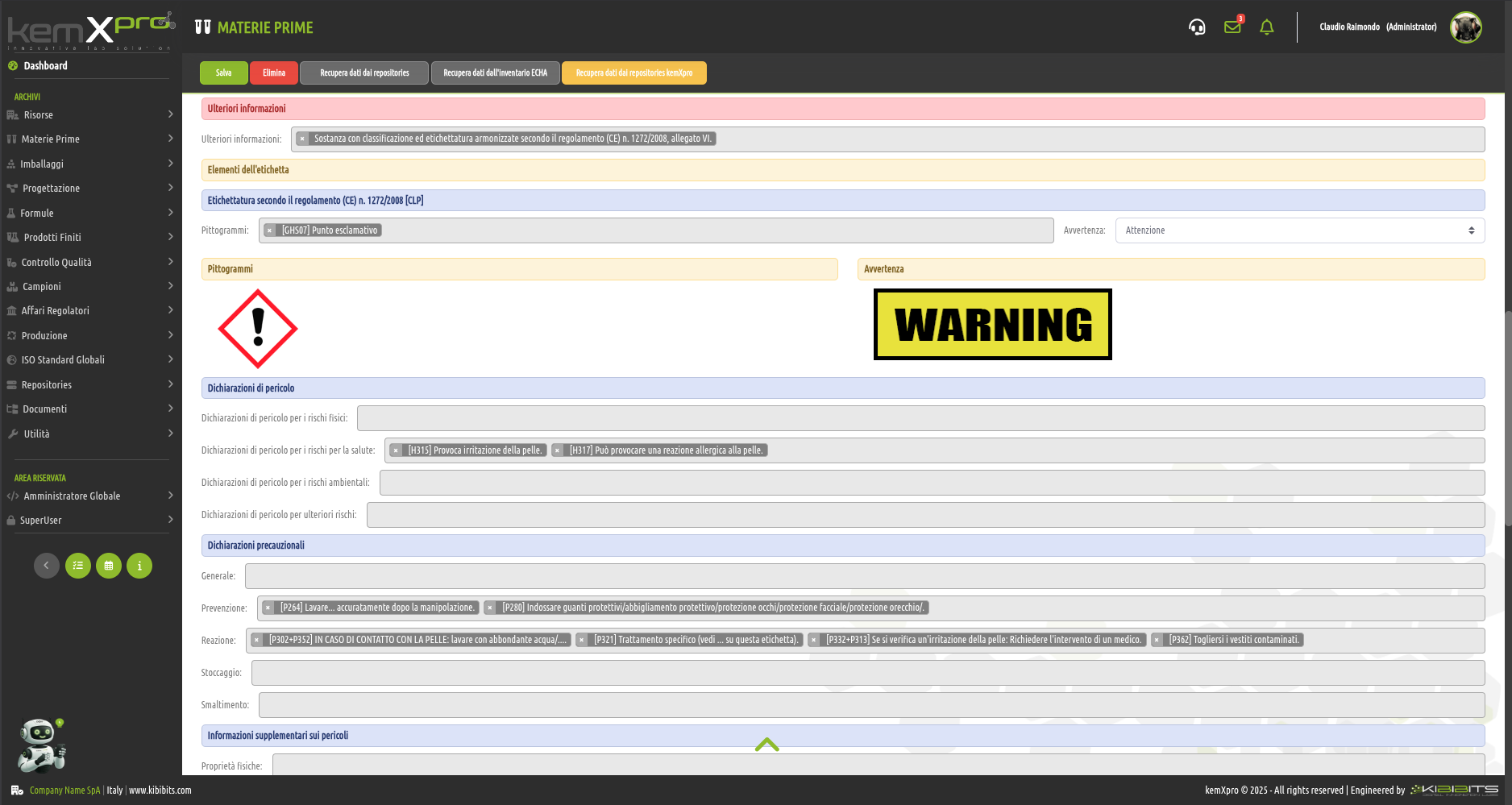
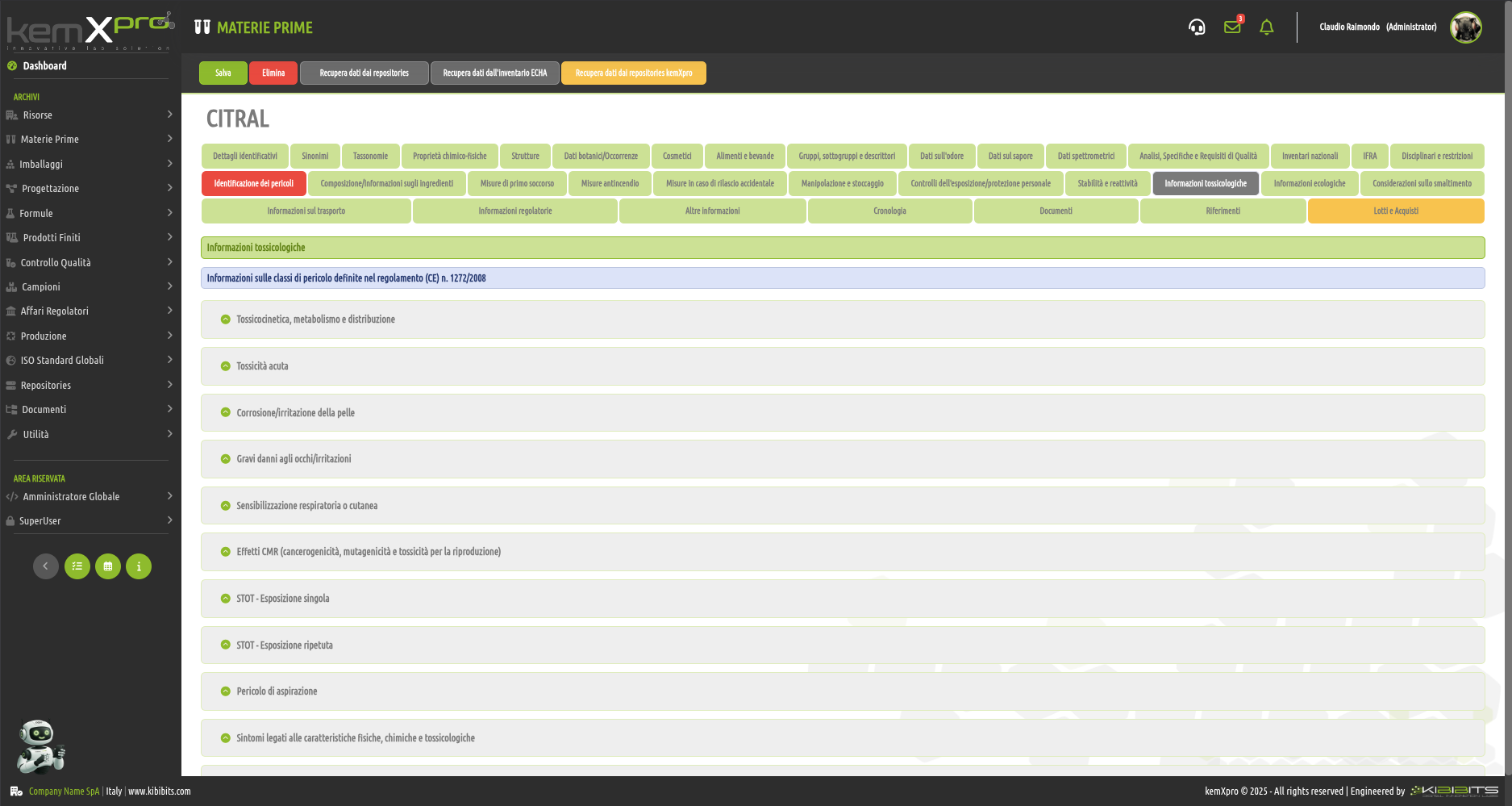
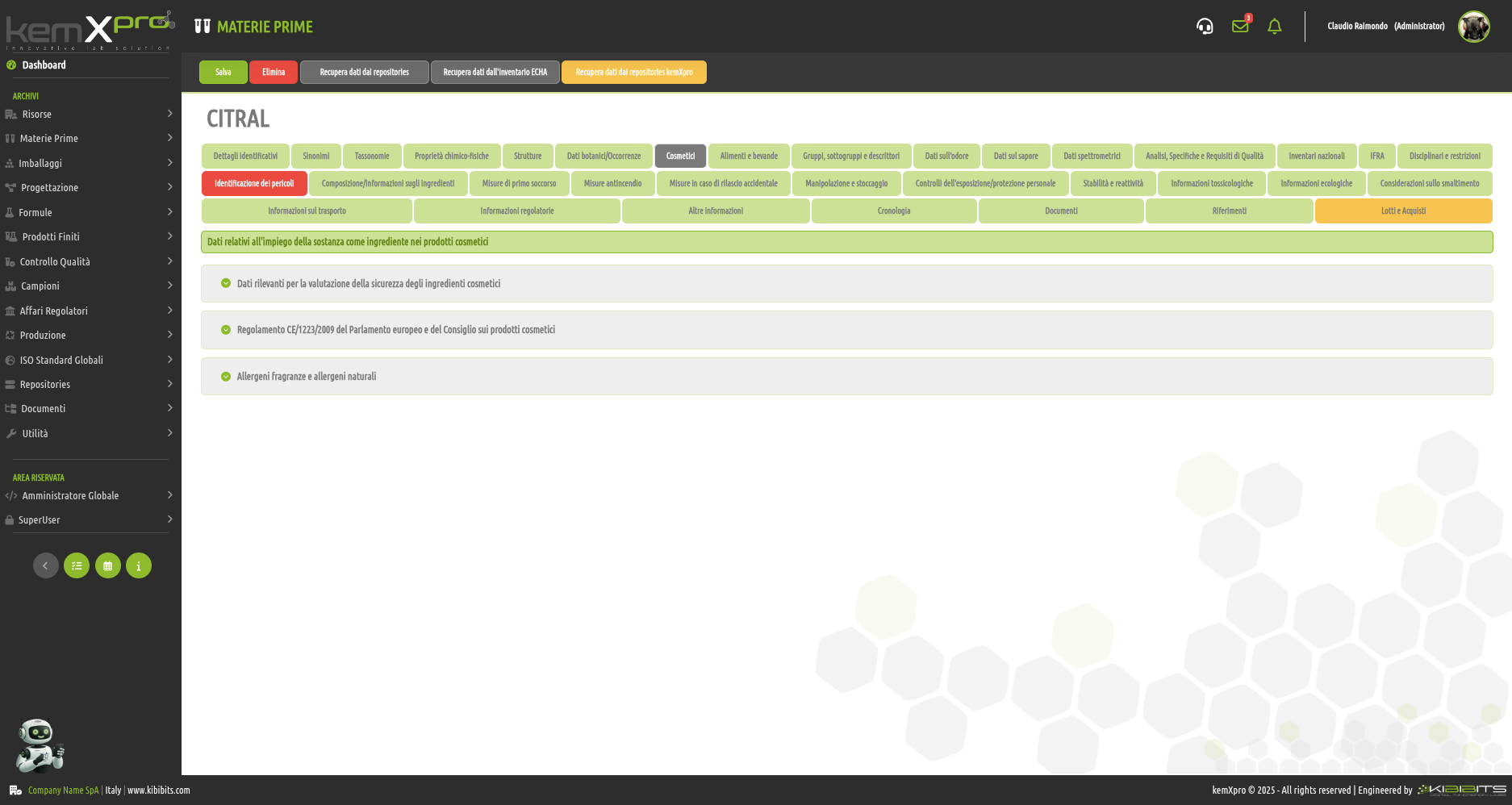
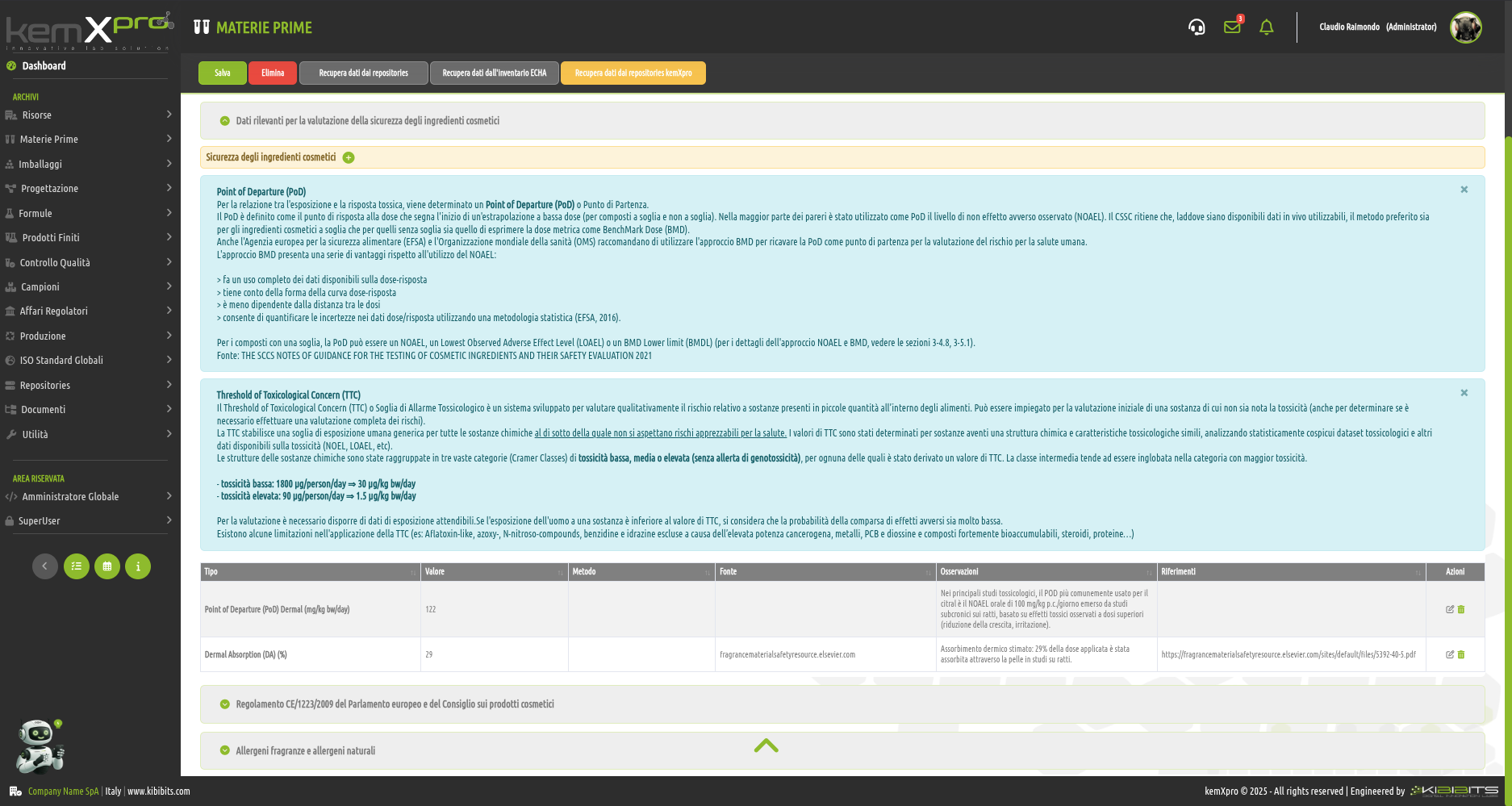
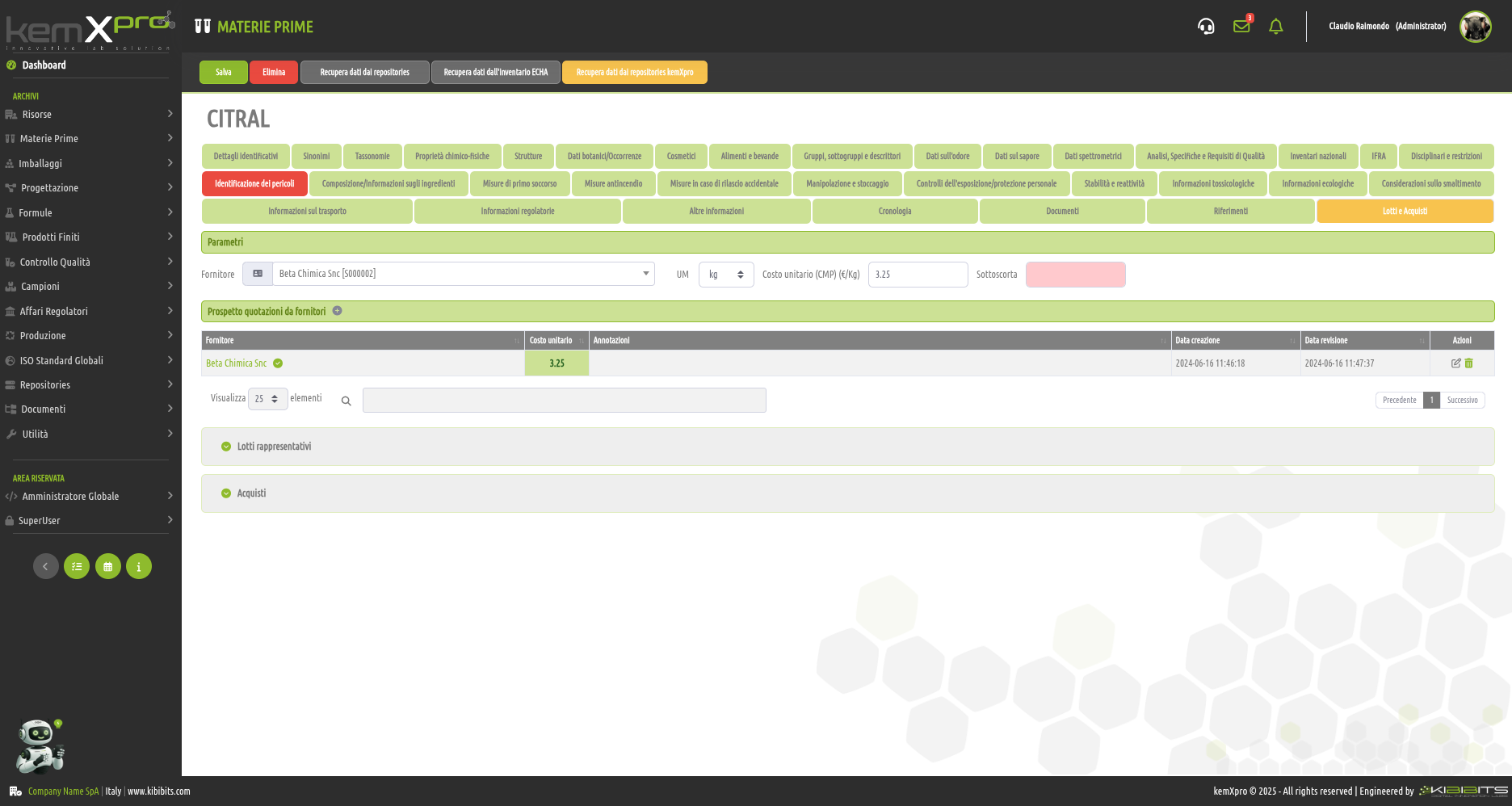
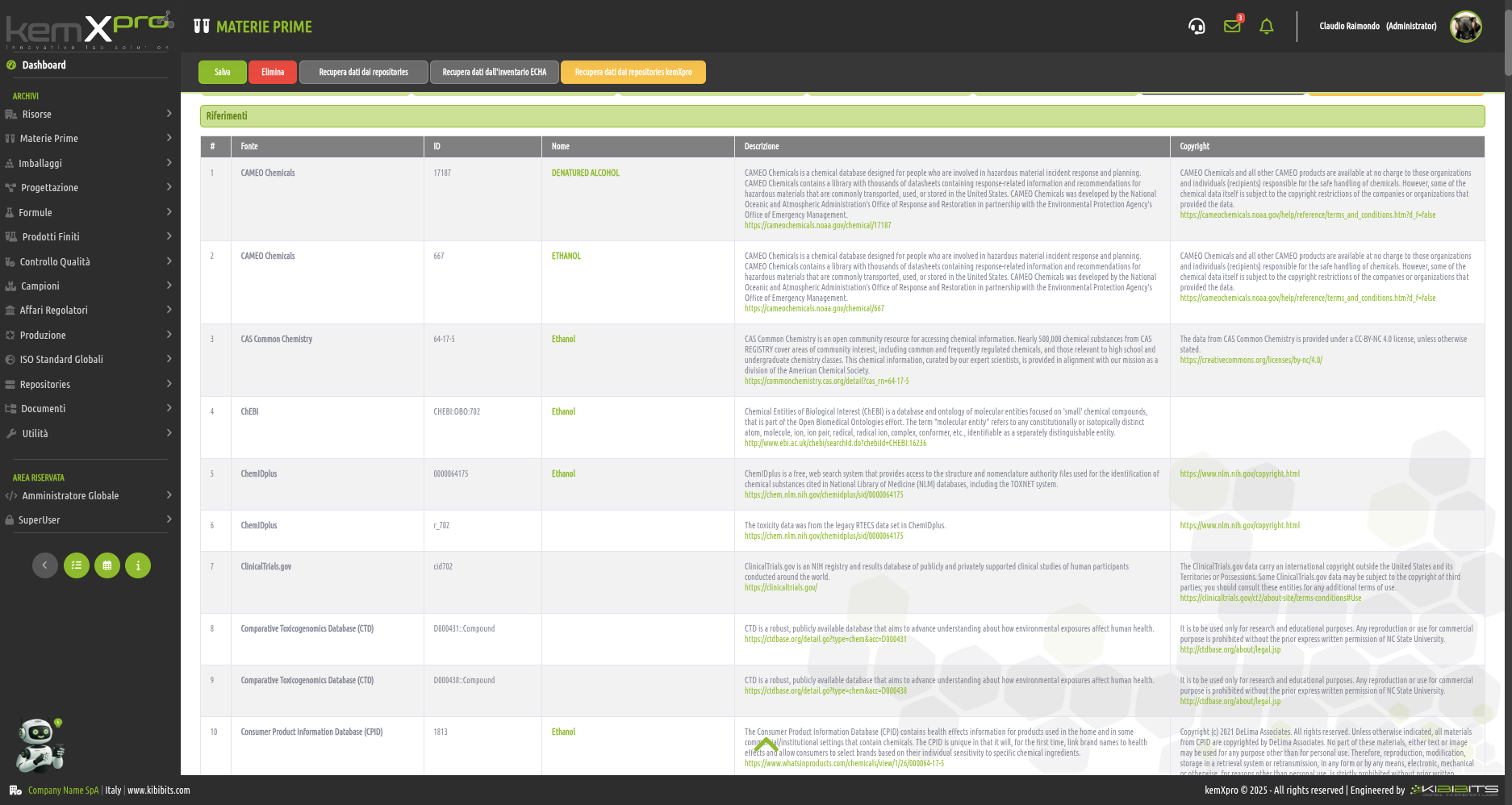
Raw Materials Management
The module provides the user with a comprehensive, practical and effective experience, and includes the ability to associate technical and regulatory information, documents, safety, storage and handling information, taxonomies, synonyms, storage, quality control, etc., with each MP.
You can create raw materials with minimal information to be integrated with technical and regulatory datasets from the main kemProd repository, containing more than 55,000 substances, or from other repositories (ECHA, IUCLID, GESTIS, PUBCHEM, etc.) to meet any compliance requirements.
It also inherits the functionality of the kemXpro IUCLID module, through which relationships can be created with the IUCLID environment, and includes advanced search and selection capabilities.


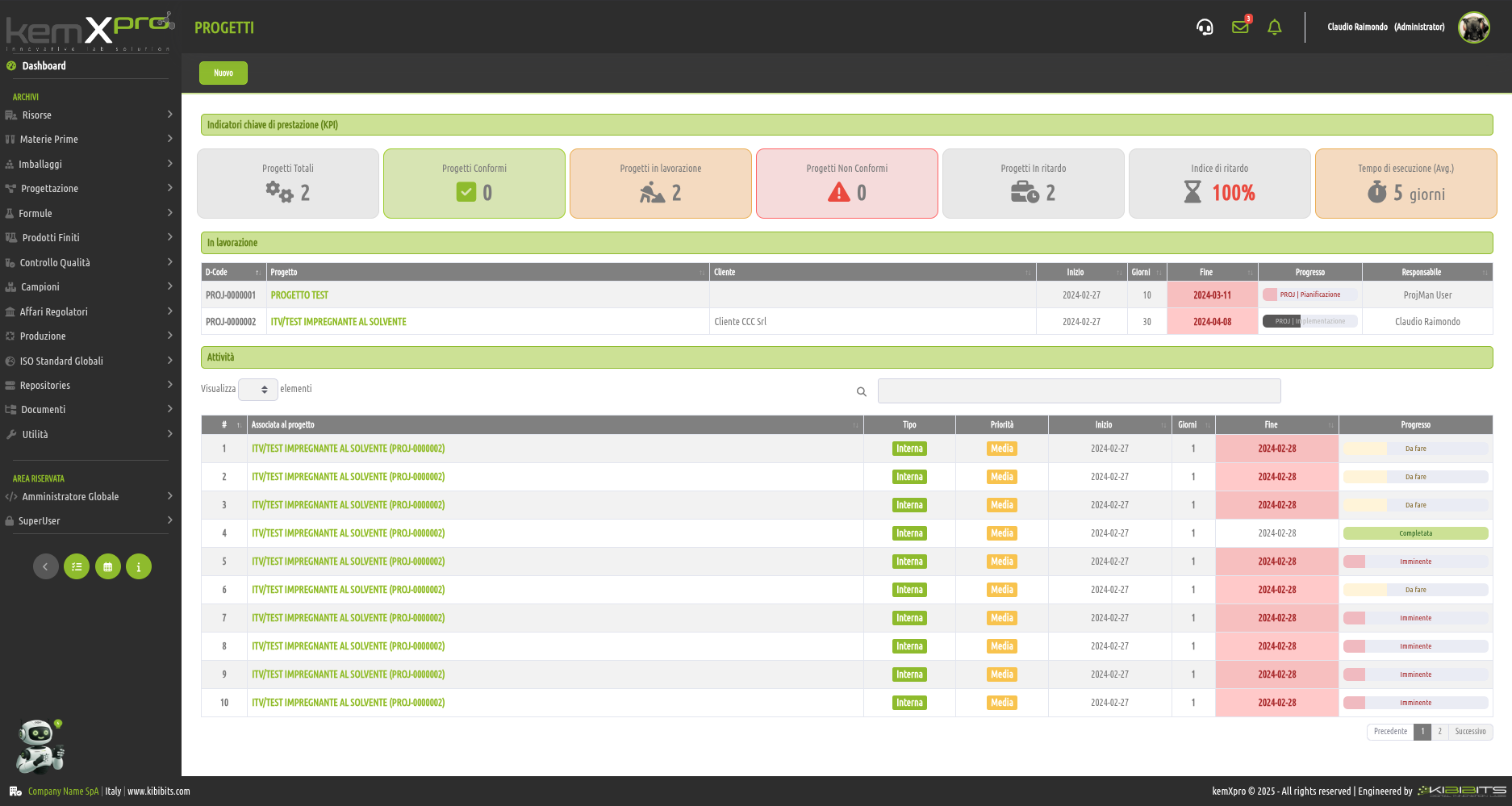
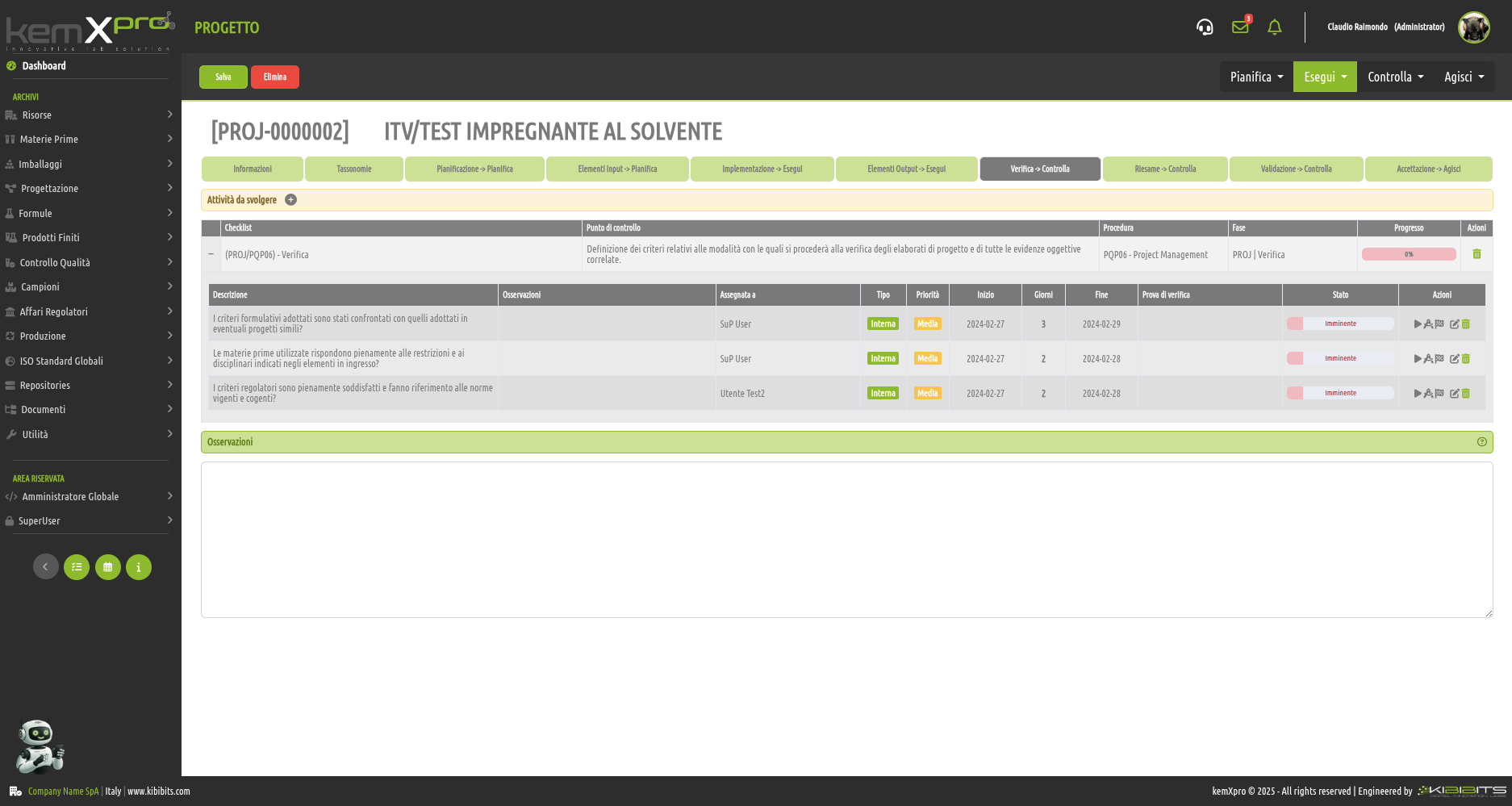
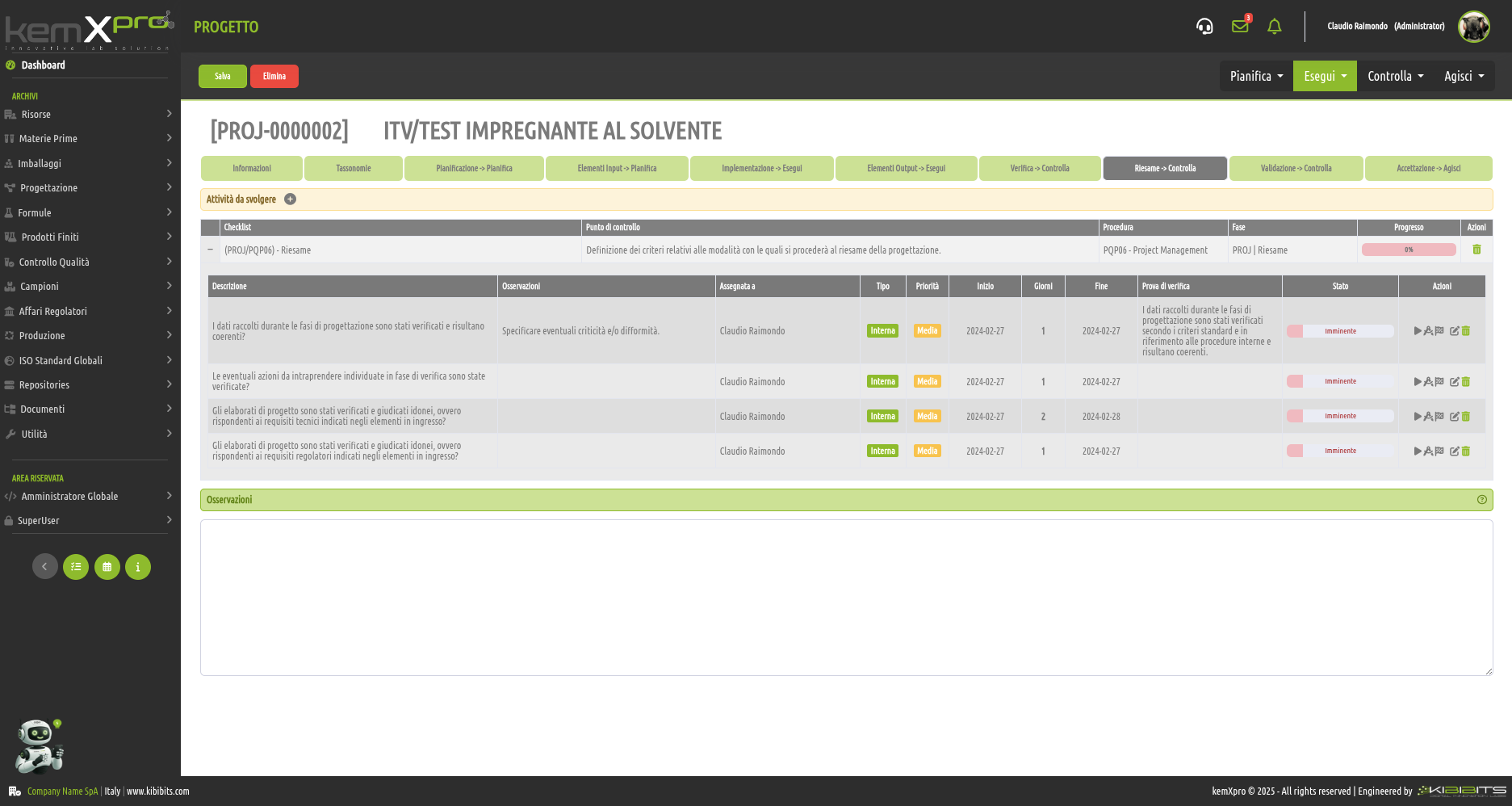
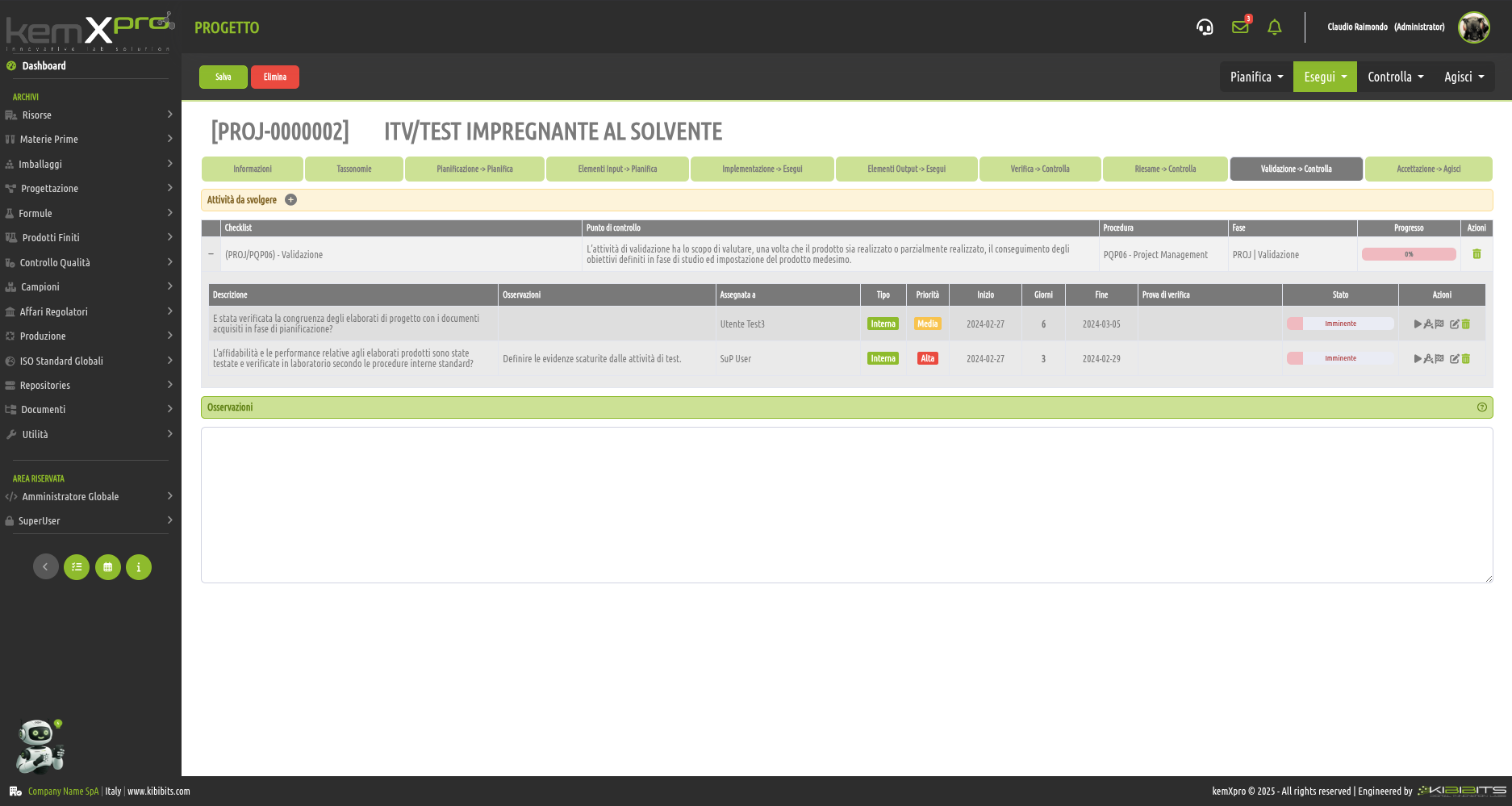
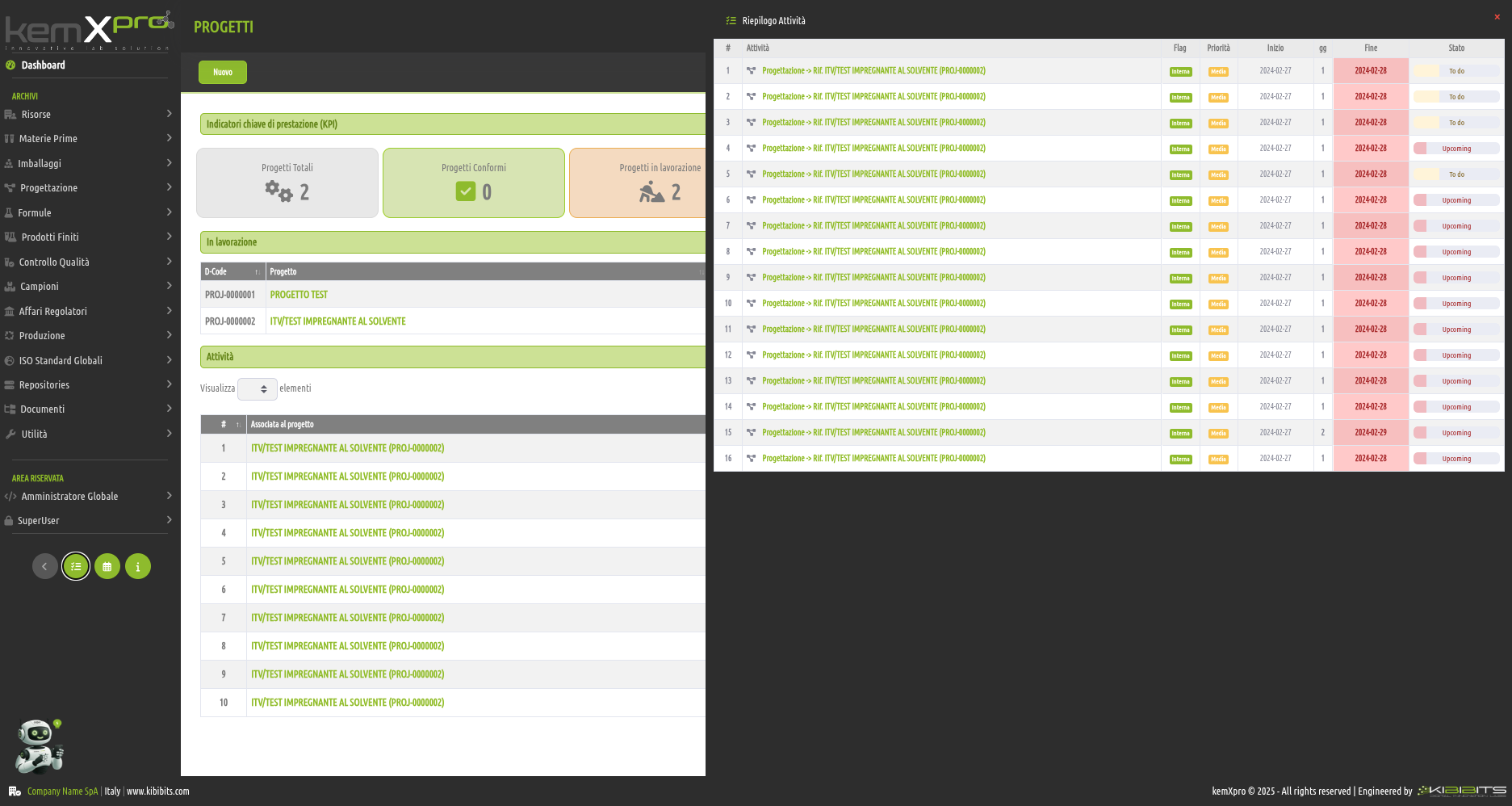
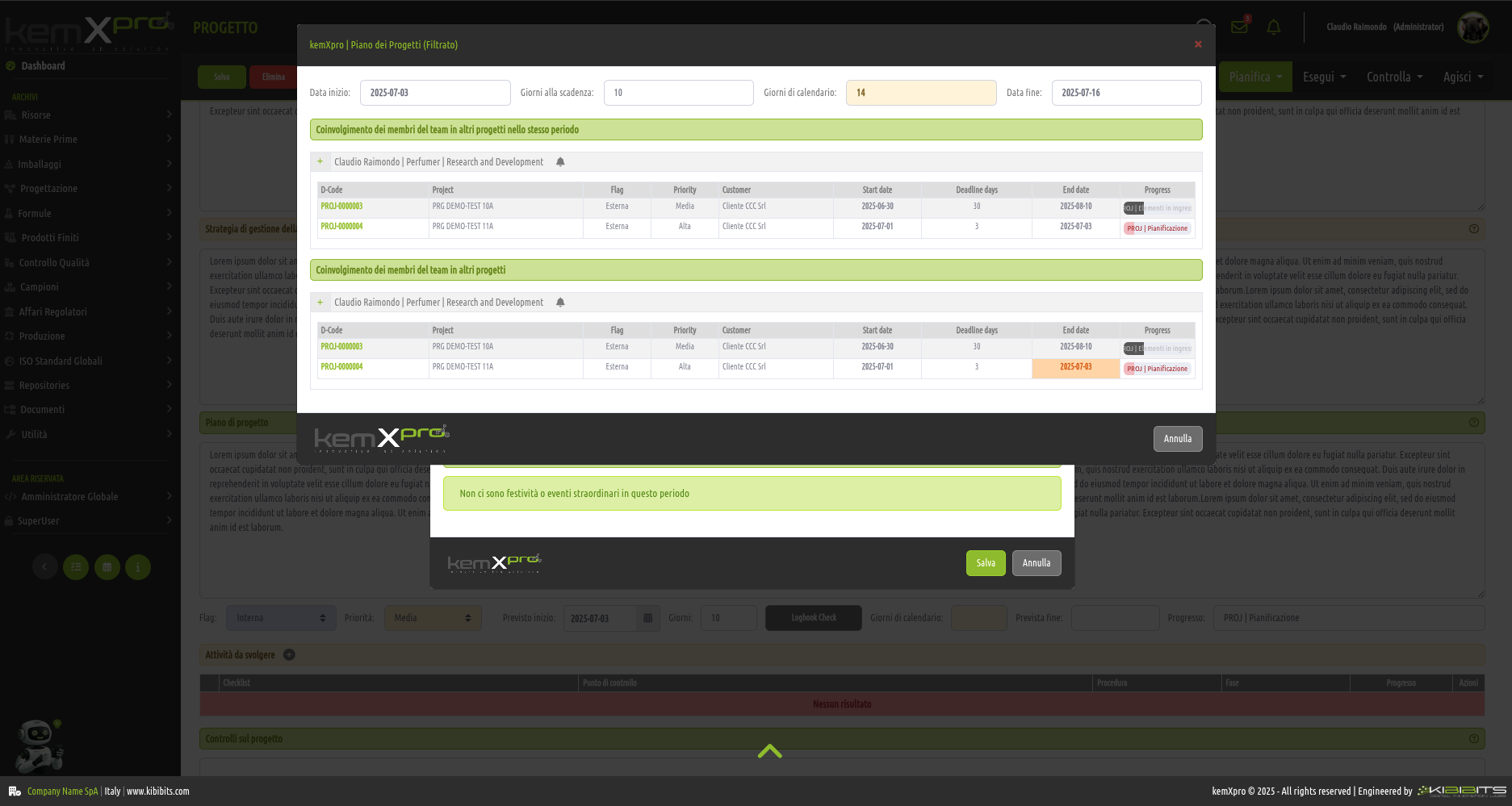
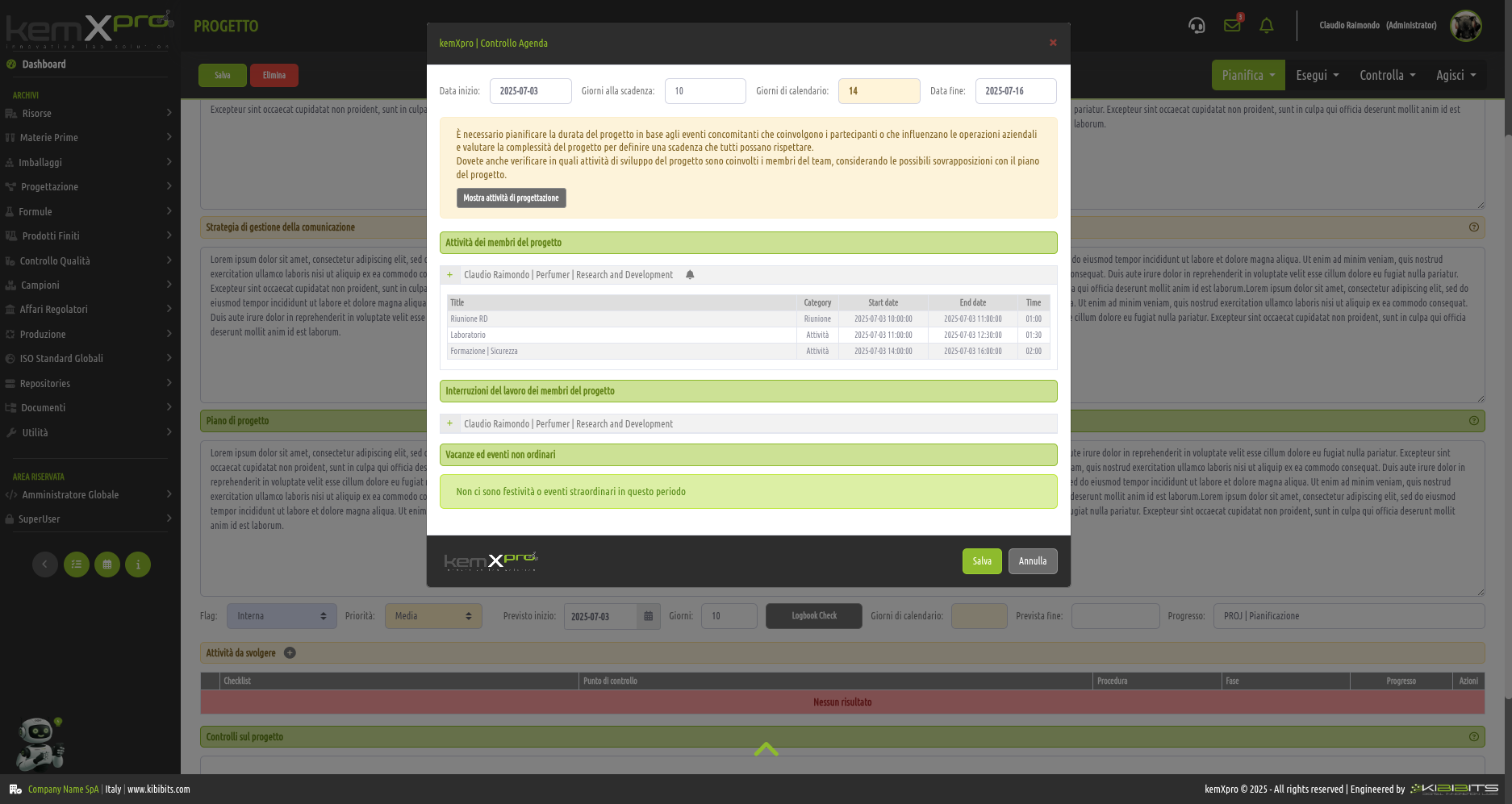
Project Management
Effective management of projects activities can only be considered a valuable asset for an organization.
The module offers the ability to easily and effectively manage the stages of analysis, planning, and implementation of project objectives, to organize in detail the activities of the personnel involved, including with reference to the check-lists provided by the Quality Management System, and to prepare clear and reliable timetables.
Smarter strategic planning and execution.




Finished Products Management
The module provides the user with a comprehensive, practical and effective experience and includes the ability to associate technical and regulatory information, documents, safety, storage and handling information, taxonomies, synonyms, samples, stock, quality control, etc.
with each reference.
It also inherits the functionality of the kemXpro IUCLID module, through which relationships can be created with the IUCLID environment, and includes advanced search and selection features.
Formulas Management
The tasks of a formulator can be multiple, often multifaceted, and require a great deal of skill and knowledge.
Formulating in full compliance with specific technical criteria and regulatory constraints, with focus on both product and process, can sometimes be complicated.
The module takes these factors into account and deploys fast and powerful computational algorithms that can clearly summarize compliance outcomes related to the configured criteria and constraints.
Convenient multilevel bills of materials allow easy management of MIMs (mixtures in mixtures), with explosion/implosion functions.
Quality Control & Quality Assurance
Quality Control plays an essential role in any production line, and making sure that the finished product meets the required quality, regulatory, and safety standards requires consistent and effective approaches, both proactive and reactive.
kemXpro allows you to define evaluation checklists and manage the activities of collecting, analyzing, and evaluating information and related documents.
It manages dispositions, communications, and authorization requests through task-lists and automatic e-mail notifications.
With kemXpro you can adequately and effectively monitor processes and control steps related to:
Supplier qualification criteria and performance monitoring
Quality of representative samples of the lots to be purchased
Quality of raw materials and batches purchased (IN)
Quality of finished products and batches sampled and/or sold (OUT)
Documentary and regulatory compliance
Traceability and retraceability
Samples Despatch
With kemXpro you can clearly and easily manage incoming (from suppliers) and outgoing (to customers) samples, associating with them the relevant technical and documentary controls to be carried out and the resulting compliance outcomes.
Handy tabs will provide you with clear and comprehensive overviews that will enable the execution of targeted monitoring activities by strategic departments, so that they can quickly and proactively make the most appropriate decisions.
Regulatory Affairs Management & Authoring
The module enables easy management of the issuance and control of regulatory documents associated with raw materials, semi-finished and finished products.
Aims to offer you a broad view regarding:
Issued documents, jurisdictions, and territorial limits
Document review status
Auditing obligations
Methods and outcomes of forwarding to users
Methods and outcomes of notifications to relevant bodies
Listed below are some of the documents that you can produce and manage:
Safety Data Sheet (SDS)
Technical Data Sheet (TDS)
Information Sheet (IDS)
Specialty Sheet (SIS)
Certificates of Analysis (CoA)
Declaration of Dermal Allergenic Substances (DASD) | Reg. 1223/2009/EC
Food Allergenic Substances Declaration (FASD) | Reg. 1334/2009/EC
IFRA Statement (IFRACD)
Product Information File | Product Information File (PIF) | Reg. 1223/2009/EC
Labels
It also inherits the functionality of the kemXpro IUCLID module, through which relationships can be created with the IUCLID environment, and includes advanced search and selection features.
Manufacturing Management
Digitization, integration and standardization of flows and processes in inventory, production, and quality operations help improve operational efficiency and transparency, increase profitability, and provide a solid foundation for continuous improvement.
Manufacturing Management defines the policies and rules necessary to maintain high levels of production and ensure that people, processes and equipment operate in a coordinated manner in accordance with required criteria.
The kemXpro Manufacturing module enables you to monitor product processing, handling, and storage processes, verify that production is taking place according to quality specifications, and gather information on resources and asset utilization to ensure that operators, supervisors, managers, and other decision makers have immediate information to effectively manage order flow and take corrective action that does not compromise quality and overall performance.
It also enables the 'optimization of workloads to be assigned to operating personnel and equipment, and manages inventories and stocks based on the planning done.
Documents Management
The kemXpro Documents module is the tool that improves productivity, increases the quality of work, and makes the passage of information smoother, facilitating more immediate sharing of documents resulting in time savings.
What benefits does it offer?
Dematerialization of documents reduces paper consumption and promotes environmental friendliness
Cuts costs associated with physically storing paper documentation
Streamlines document workflow and reduces document search and transmission time
Makes documents unalterable and always available
Allows users to consult the same material at the same time
Reduces management costs, streamlines internal processes, and increases efficiency and competitiveness by reducing the risk of errors
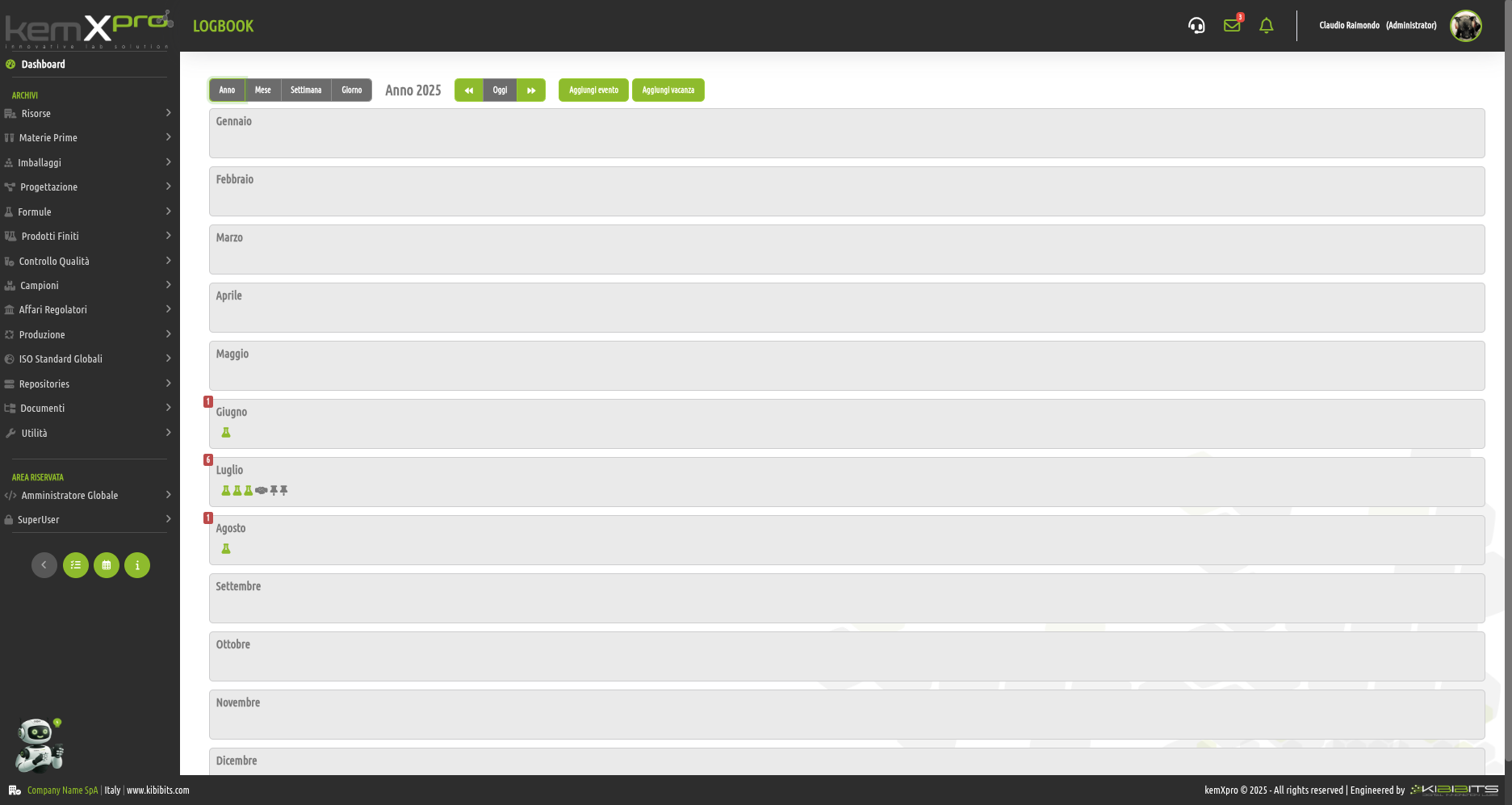
Activities and events
Leveraging technology to monitor the commitments, schedules and workloads that burden human resources in your organization is a''logical extension of what you are already doing to improve productivity.
Rushed deadlines and busy schedules often lead to mistakes, sometimes serious ones, generate fatigue, frustration, and loss of efficiency and motivation.
Knowing "who is doing what? and for how long? with reference to what procedure?" is a great advantage that allows you to have a constant focus on operational performance, goal achievement and proper execution of processes.
The kemXpro Logbook module can offer you valuable help by automatically generating agendas and logs that enable you to ensure high levels of engagement and performance with a view to constant and continuous improvement.
Managers and supervisors will be able to plan and organize activities according to actual operational availability and set respectable deadlines based on realistic timelines.
BoardLink Instanting Messages
Why unnecessarily overload email servers when you can offer your employees the ability to interact easily and with immediacy?
With kemXpro BoardLink you can have as simple as effective features to make operations smoother and lighten the overall load on your infrastructure.
Chron Jobs Management
Scheduling scheduled events is possible thanks to the kemXpro ChronJobs module.
For various reasons you may need to perform, in a fully automatic mode, periodic functions of calculation and control, data import and export, document issuances and revisions or other.
We will analyze criteria and needs with you and be able to provide you with the most effective solution.
Languages Management
The kemXpro Languages module, based on neural translation technology, currently contemplates the integrated management of more than 100 languages for documents and GUI (Graphical User Interface).
The scalability features of kemXpro allow you to install them according to current need and integrate them according to future needs, including to materialize useful data distribution and resource utilization.
Taking advantage of AI (Artificial Intelligence), especially the ML (Machine Learning) method of analysis, kemXpro Languages can come to your aid in case you need to train customized translation models to meet specific needs.
SuperUser Management
The kemXpro SuperUser module allows the SuperUser to have a clear focus on the workgroup and to create and assign specific roles and permissions to each user, in relation to the installed modules.
While roles are created and assigned with reference to the company's organizational chart, permissions are sets of rules that determine the type of interaction a role can have toward other users and business elements.
By virtue of this, they are designed to help you manage what users are allowed to see or do within kemXpro, with reference to the CIA Triad and the criteria set forth in corporate policies.
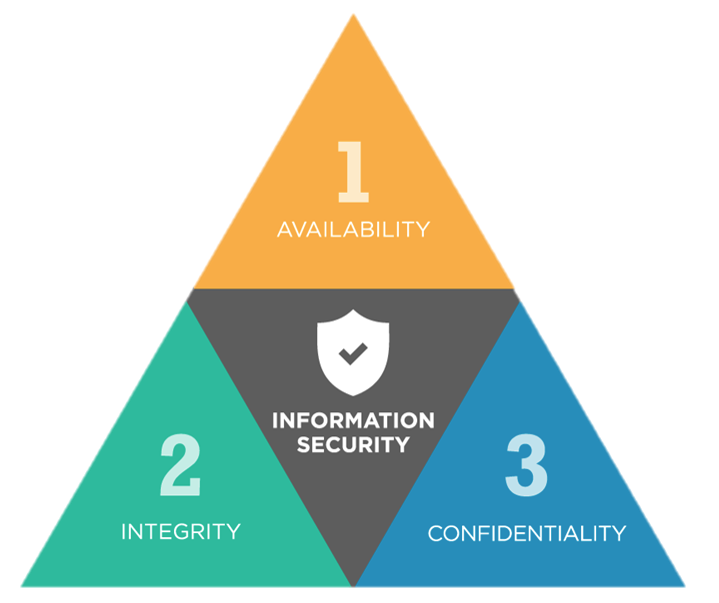
The CIA triad (Confidentiality, Integrity, Availability) is considered the foundation of information security.
Availability (Availability)
Authorized users have access to the systems and resources they need.
Integrity (Integrity)
Data are protected from unauthorized modification to ensure that they are reliable and correct.
Confidentiality (Confidentiality)
Data, objects and resources are protected from unauthorized viewing/exporting/printing.
In general, the 'array of permits refers to:
POST → Creation
GET → Reading
PUT → Upgrade/Replacement
PATCH → Update/Modification
DELETE → Elimination
PRINT → Print
EXPORT → Export
Flavours and Fragrances
Designed and built for creators and producers of flavors and fragrances, aromatherapists and herbalists.
The kemXpro Flavours & Fragrances module integrates powerful computational algorithms to field advanced features geared toward practical, comprehensive, automatic, immediate, and effective formula development and for high-level technical and regulatory compliance assessment.
Advanced tools, based on state-of-the-art technology for data generation, modeling, and analysis, optimize and facilitate the process of raw material selection and formula development, and guide a proactive approach to achieving consistent performance.
Intelligent generation based on sensory profile models can also provide valuable support in specific use cases.
What it can mean. Design your own "olfactory model," and kemXpro will take care of the rest.
Raw materials and semi-finished products classified and classifiable by reference to:
Intensity, volatility and substantivity
Time bands
Descriptions of smell and taste
24 groups, 78 subgroups and 419 descriptors/triggers referred to odor and taste
Odour Threshold Values and usage suggestions in fragrances
Taste Threshold Values and usage suggestions in flavors
Occurrences in nature and NCS (Natural Complex Substances) correlations
Botanical data
A repository containing over 15,000 entries referring to medicinal herbs and essential oils can provide you with useful information on specific characteristics and composition.
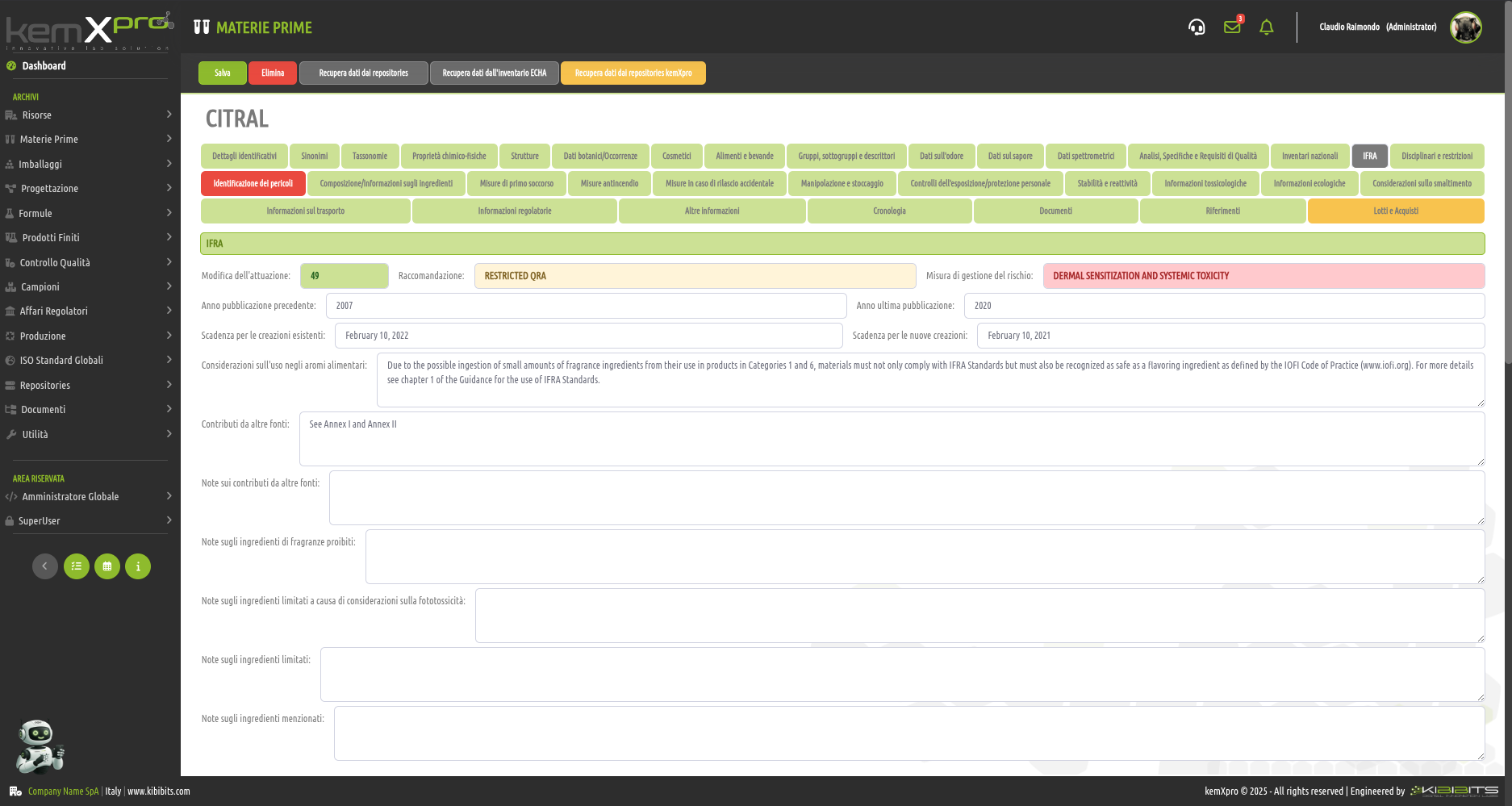
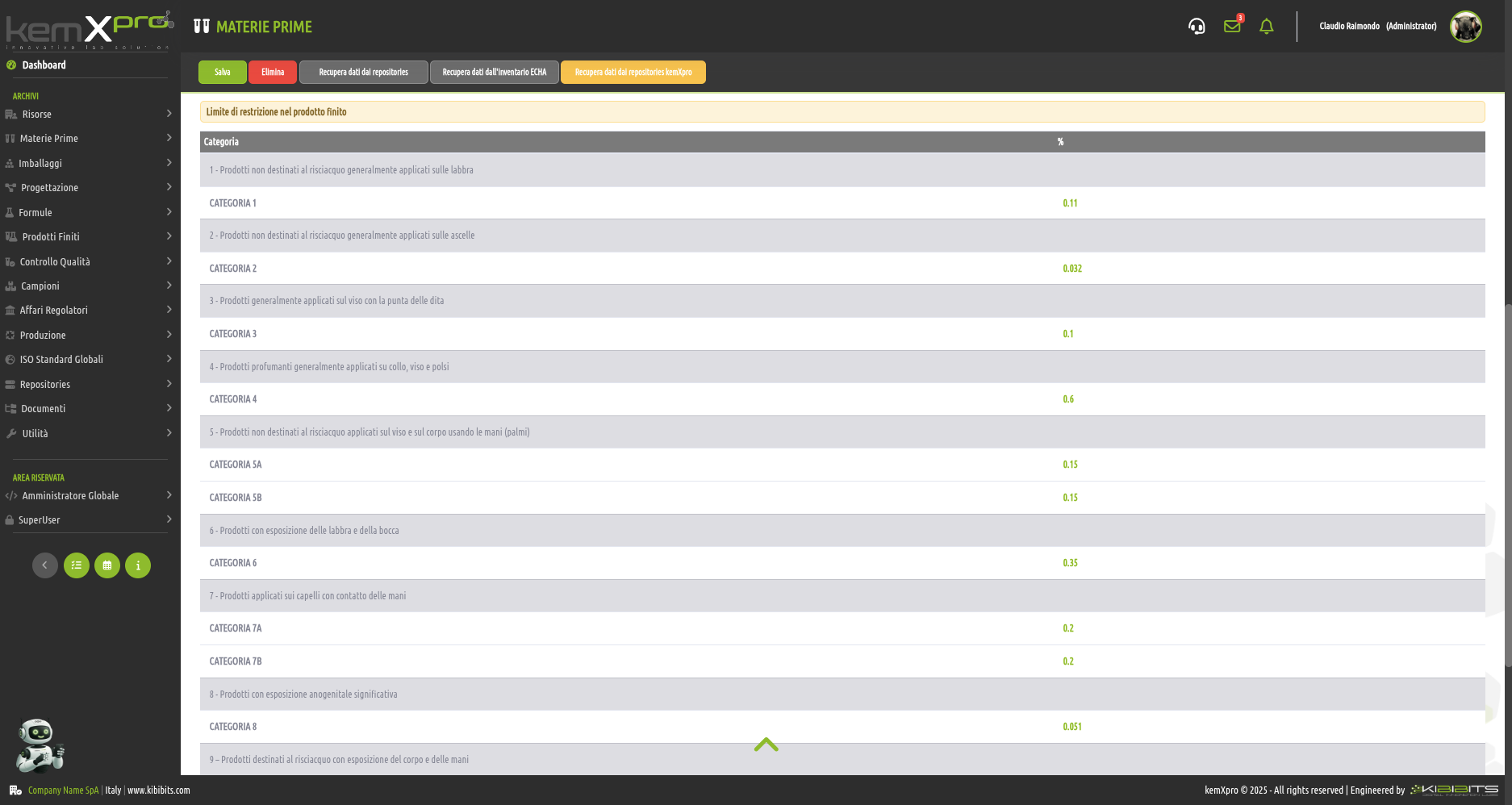
IFRA Management for Flavours and Fragrances
International Fragrance Association (IFRA), thanks to an impeccable team of experts, has been setting IFRA standards for over forty years to promote the''safe use of fragrances and ensure that people can benefit from the''art of perfumery with confidence.
If IFRA did not exist, it would certainly have to be invented!
The kemXpro IFRA module enables the integration of comprehensive datasets and advanced, fully automated computational functions to provide technicians and formulators with practical and comprehensive global compliance assessments of flavors1 and fragrances.
(1) Because of the possible ingestion of small amounts of fragrance ingredients due to their use in Category 1 and 6 products, the materials must not only comply with IFRA standards, but must also be recognized as safe fragrance ingredients, with reference to what is defined by the Code of Practice IOFI, and authorized by the jurisdiction regulating the intended sales market.
Safety Data Sheet (SDS) | Automated Authoring System (AAS)
The kemXpro SDS-AAS module generates SDSs easily and effectively due to the integrated legal content and automatic classification.
It has been implemented with reference to the EuPhraC (European Standard Phrase Catalogue) catalog (SDSCom + ESCom XML data exchange SDS + exposure scenario).
EuPhraC is an independent, non-proprietary standard phrase catalog that defines a set of appropriate and legally compliant phrases to be adopted for document communications along the supply chain.
It is aligned and designed to support SDScomXML and includes all ESCom phrases for exposure scenarios, thus enabling extended safety data sheets to be properly compiled.
It also leverages additional taxonomic and descriptive phrases, containing more than 50,000 phrases translated into more than 50 languages, to extend content and expressive capabilities.
Documents can be generated in accordance with:
UN GHS | United Nations GHS System (UNECE)
REACH/CLP | EU Implementation of GHS
US OSHA HCS | 29 CFR 1910.1200 Appendix D
National GHS implementations already integrated into kemXpro
The entry of the necessary information and a handful of clicks are required to:
create "draft" documents to be released when you have completed the verification steps
generate automatic classifications to be modified freely if necessary
translating documents into more than 30 languages
review and issue new documents when circumstances arise that require it
scheduling authoring activities
distribute documents in full compliance with laws and with minimal effort
GHS USA (OSHA) | OSHA Hazard Communication Standard (HCS) (29 CFR 1910.1200)
GHS Canada (HPR) | Hazardous Products Regulations (HPR)
GHS Mexico | NOM-018-STPS & NMX-R-019-SCFI
GHS UN | United Nations (UN) Model Regulation: Globally Harmonized System of Classification and Labeling of Chemicals
GHS EU CLP/REACH | Classification, Labeling and Packaging of Substances and Mixtures (1272/2008/EC). Registration, Evaluation, Authorisation and Restriction of Chemicals (1907/2006/EC)
GHS Brazil | Brazilian Standard ABNT NBR 14725
GHS Australia (WHS Regulations) | Safe Work Australia | Model Work Health and Safety (WHS) Regulations
GHS China | National Standard GB 13690 | State Council Decree 591 | Regulations on Safe Management of Hazardous Chemicals
GHS Taiwan | Taiwan National Standards CNS 15030
GHS Japan | Japanese Industrial Standards JIS Z7252
GHS Korea | MOEL Public Notice No. 2016-19
GHS Indonesia | Peraturan Nomor 04/BIM/PER
GHS Thailand | B.E. 2555
GHS Malaysia | Peraturan CLASS/CLASS Regulations
GHS Serbia
GHS Singapore | Singapore Standard SS 586
GHS Russia | GOST 32419 | Classification of Chemicals, general requirements and other standards
GHS Turkey | T.C 28848 | SEA Regulation
GHS GB | GB CLP/UK REACH
NFPA® 704 | Standard System for the Identification of the Hazards of Materials for Emergency Response according to NFPA (National Fire Protection Association)
HMIS® | Hazardous Materials Identification System according to ACA (American Coatings Association)
ADR/RID/ADN Road/Rail/Waterway | European Agreements concerning the International Carriage of Dangerous Goods by Road/Rail/Inland Waterways (ADR/RID/ADN)
IMDG-Code Sea | International Maritime Dangerous Goods Code
ICAO/IATA Air | International Civil Aviation Organization, International Air Transport Association, Dangerous Goods Regulations
DOT (USA) | 49 CFR § 40 U.S. Department of Transportation
UN RTDG Orange Book | United Nations (UN) Recommendations on the Transport of Dangerous Goods
Occupational Exposure Limit Values (OELV) | EU | National for all country packages, ACGIH TLV®, etc.
Biological Limit Values (BLV) | EU | National limit values, ACGIH BEI®, etc.
Carcinogenic, Mutagenic and Reprotoxic Chemicals (CMR) | EU | National limit values, TRGS 910, SZW-Lijst, Proposition 65, OSHA, NTP, IARC, etc.
CERCLA | USA | Comprehensive Environmental Response, Compensation and Liability, Hazardous Substances and Reportable Quantities (RQ)
Clean Air Act | USA | Accidental release prevention
Right to Know Acts | California, Minnesota, Massachusetts, Rhode Island, Pennsylvania, New Jersey, USA | Hazardous Substance Lists
SARA 355 | USA | Extremely Hazardous Substances
SARA 313 | USA | Specific Toxic Chemical Listings
REACH Annex XVII | Regulation EU | Restrictions on the manufacture, marketing and use of certain dangerous substances, mixtures and articles
SVHC REACH Annex XIV | Regulation EU | Substances of Very High Concern
POP | Regulation EU | Persistent Organic Pollutants (850/2004/EC)
PIC | Regulation EU | Export and import of dangerous chemicals subject to the PIC-procedure (Prior Informed Consent) (649/2012/EU)
PRTR | Regulation EU | Pollutant release and transfer register (166/2006/EC)
EDC | Regulation EU | Substances that interfere with the hormone system
RoHS | Directive EU | Restriction of use of certain hazardous substances in electrical and electronic equipment (2011/65/EU)
Ozone | Regulation EU | Substances that deplete the ozone layer (1005/2009/EC)
SEVESO | Directive EU | Control of major-accident hazards involving dangerous substances (2012/18/EU)
Dual-Use | Regulation EU | Community regime for the control of exports, transfer, brokering and transit of dual-use goods (428/2009/EC)
Drug-Precursors | Regulations and int. Convention EU, USA and UN | Regulations to prevent illicit traffic in narcotic drugs and psychotropic substances and drug precursors
Explosive-Precursors | Regulation EU | Marketing and use of explosives precursors (98/2013/EU)
Biocides | Regulation and Directive EU | Placing on the market and use of biocidal products and placing of biocidal products (528/2012/EU)
Detergents | Regulation EU 648/204/EC
VOC Deco-Paint and Industrial Emissions | Directives EU | Limitation of emissions of volatile organic compounds (VOCs) due to the use of organic solvents in certain paints and varnishes and integrated pollution prevention and control (VOC emissions by industry) (2004/42/EC and 2010/75/EU)
VOCV | Switzerland | Incentive tax on volatile organic compounds
LGK Storage Class TRGS 510 | Germany | Storage of hazardous materials in portable containers
Water Framework Directive, WFD | Directive EU | Framework for community action in the field of water policy (2000/60/EC)
WGK Water Hazard Class AwSV | Germany | Regulation on systems for handling water-polluting substances (Verordnung über Anlagen zum Umgang mit wassergefährdenden Stoffen)
ABM Water | Netherlands | General Assessment Methodology (Algemene Beoordelings Methodiek)
TA Luft | Germany | Technical Instruction on Air Quality Control (Technische Anleitung zur Reinhaltung der Luft)
VbF | Austria | Regulation on flammable liquids (Verordnung über brennbare Flüssigkeiten)
Hazchem | Emergency Action Code (EAC) | United Kingdom, Australia, New Zealand, Malaysia, India
Waste Catalogues Directive and List EU and Basel Convention
GADSL Global Automotive Declarable Substance List
TSCA | USA | Toxic Substances Control Act
REACH | EU | REACH Regulation
DSL/NDSL | Canada | Domestic and Nondomestic Substances List
CSCL/ISHL | Japan | Chemical Substance Control and Industrial Safety and Health Law
NZIOC | New Zealand | Inventory of Chemicals
IECSC | China | Inventory of Existing Chemical Substances
TCSI | Taiwan | Chemical Substance Inventory
AICS | Australia | Inventory of Chemical Substances
EINECS, ELINCS, NLP | EU | EC Chemical Substances Inventories
KECL/KECI | South Korea | Existing Chemicals List
PICCS | Philippines | Inventory of Chemicals and Chemical Substances
INSQ | Mexico | National Inventory of Chemical Substances
A socket for IUCLID Environment
IUCLID is the essential tool for any organization or individual who needs to record, store, submit and exchange chemical data in the format of the OECD (Organization for Economic Co-operation and Development) harmonized templates.
The kemXpro IUCLID module is designed to make data-mining and data-exchange processes to and from the framework easier and more immediate and to minimize redundant and time-wasting entries.
You will not need to create any files or perform any manual operations, just one click.
You can create UUID associations and import and export:
Legal entities
Contact
Production sites
Substances
Reference Substances
Mixtures
Compositions
Chemical and physical properties
Hazard classifications
Dossier and related information
kemXpro kemIA Virtual Assistant
#NOTF[02.180]#
Why might you choose it?
kemXpro is an expression of cutting-edge technologies that enable you to innovate, experiment, compete, design, support, and anticipate.
Whether your company is at the beginning or well on its way to digital transformation, kemXpro can help you solve your toughest challenges.
It's a web application with a practical and intuitive interface that enables easy interaction, geared toward consistent and highly efficient management of R&D, Products and Quality Management activities and commitments in regulatory affairs.
A scalable container of services, data and technologies that can easily adapt to processes by pandering to the needs of strategic departments.
Raw materials, formulas, activities, research and development, product quality, samples, technical and regulatory documents, safety data sheets, specifications, compliance criteria, data-mining, legal compliance, sharing and interoperability are no longer a problem but an opportunity.
By professionals, for professionals!


Learn more...
In our YouTube channel you will find video tutorials, hands-on use demonstrations, and in-depth case studies that can provide you with additional evaluation elements or can support you in using specific functions.
Go
Want to see kemXpro in action? Contact us for a live demo!
We will be happy to organize an online demonstration session where you can focus, with the support of our technicians and developers, on the specific features of kemXpro and how it could fit the needs related to your management model.
Participation is free and you will not have to move from your office.



